Migraine-associated genes, activation of specific brain regions and cervical nerves, and neuropeptides are all contributory pathophysiological factors for migraine and have led to the development of a variety of novel mechanism-based therapies. Can these therapies be applied equally to both episodic and chronic migraine or do episodic and chronic migraine have distinct pathophysiologies? asked Professor Andrew Charles, David Geffen School of Medicine at UCLA, LA, at the 2019 Scottsdale Headache Symposium.
In the future, it will be possible to tailor treatments based on the distinct mechanisms of migraine that affect individual patients,1 predicted Professor Andrew Charles. Understanding the precise mechanisms involved for each patient is key, and then translating them into clinical practice in the diagnosis and treatment.
Multiple pathways could be involved in both episodic and chronic migraine
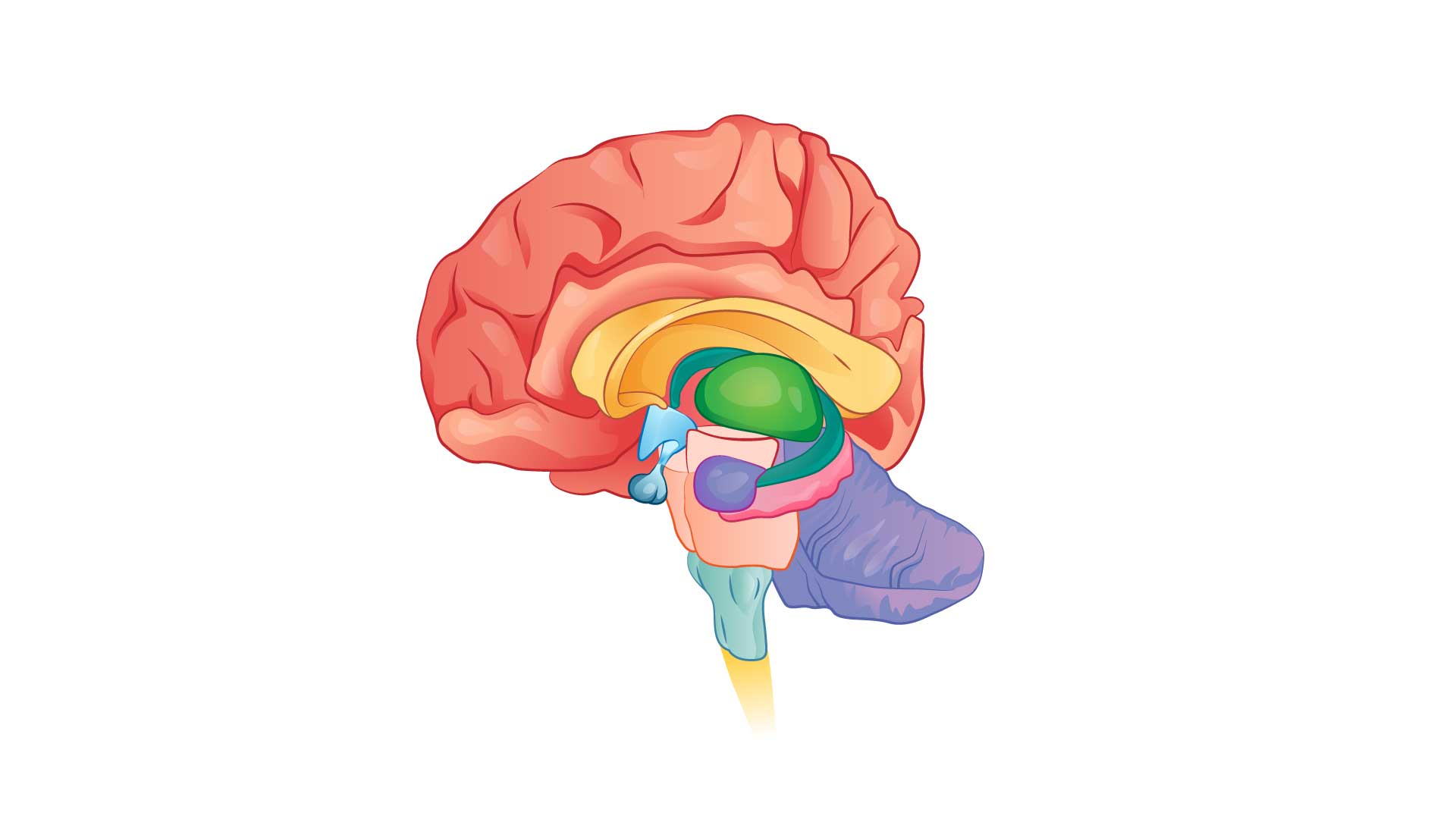
Professor Charles highlighted the complex pathophysiological processes associated with migraine. Multiple genetic, environmental, hormonal, metabolic and iatrogenic triggers result in diffuse neurochemical and neurophysiological alterations, which in turn lead to specific central and peripheral nervous system dysfunction and neuropeptide release ultimately leading to a migraine attack with or without aura.1
Multiple pathways could be involved in both episodic and chronic migraine, and plasticity and neuromodulation also need to be considered, said Professor Charles. A migraine attack is a complex neurological event taking place over hours to days involving the cortex, thalamus, hypothalamus, upper cervical nerves and trigeminocervical complex as well as the release of calcitonin gene related peptide (CGRP) and pituitary adenylate cyclase-activating polypeptide (PACAP).1
Some genes predispose to chronic migraine
Genetic polymorphisms are associated with earlier onset and more severe migraine
Although some genes predispose to chronic migraine, Professor Charles suggested that chronic migraine is part of a migraine spectrum. At least 38 genetic polymorphisms are associated with migraine and are associated with earlier onset and more severe attacks. He suggested that a gene panel might predict chronification of migraine requiring different treatment.2
Are gray matter changes of chronic migraine, a cause or a consequence?
Chronic migraine is associated with altered connectivity of the anterior insula, amygdala, pulvinar, mediodorsal thalamus, middle temporal cortex and periaqueductal gray matter.3 Furthermore, Patients with migraine, particularly those with chronic migraine, have decreased gray matter density or volume in structures in the pain matrix involved in descending pain inhibitory modulation of pain — that is, the cingulate cortex, insula, prefrontal cortex, amygdala, parietal cortex, and superior temporal gyrus and temporal pole,4 said Professor Charles. But, it is not clear whether these changes are a cause or a consequence of migraine. Are the functional changes specific to migraine or a reflection of chronic pain? he wondered, and he noted that the changes are reversible reflecting brain plasticity.
Medication overuse migraine
Changes associated with medication overuse are reversible
A very large proportion of chronic migraine can be attributed to medication overuse headache. Medication overuse headache is associated with reversible changes in the metabolism of pain processing structures. Changes include hypometabolism in the bilateral thalamus, orbitofrontal cortex (OFC), anterior cingulate gyrus, insula/ventral striatum, and right inferior parietal lobule, and hypermetabolism in the cerebellar vermis. All dysmetabolic areas except the OFC recover to almost normal metabolism after analgesic withdrawal.5
CGRP levels in chronic migraine
CGRP levels are raised in chronic migraine
CGRP plays a key role in migraine, said Professor Charles. It is released during a migraine attack, levels are elevated in chronic migraine, CGRP administration triggers migraine, small molecule CGRP antagonists abort migraine, and antibodies to CGRP or its receptor prevent migraine6.
Interictal increase in CGRP levels in peripheral blood could be a biomarker for chronic migraine,7 but due to technical challenges are not yet used as a biomarker, said Professor Charles. CGRP assays are difficult to carry out and produce inconsistent results.
A variety of environmental and physiology “danger” signals activate CGRP neurons — could this be the mechanism for migraine triggers? Professor Charles wondered, and he highlighted that many questions remain about CGRP — Where is it released? Which cells release it? Where is its most important site of action in migraine? Is the action in the peripheral or central nervous system? and If CGRP is persistently elevated in migraine, why?
Our correspondent’s highlights from the symposium are meant as a fair representation of the scientific content presented. The views and opinions expressed on this page do not necessarily reflect those of Lundbeck.