Brintellix® (vortioxetine) improves major depressive disorder (MDD) symptoms (1,2)
A study by Alvarez et al. 2012 demonstrates Brintellix® as an efficacious antidepressant for the management of depressive symptoms in patients with MDD2
In this double-blind, randomized, placebo controlled, active reference study,
Brintellix® demonstrated effective relief from symptoms of MDD at 6 weeks (MADRS ≥ 30).2
Explore the data in more detail below.
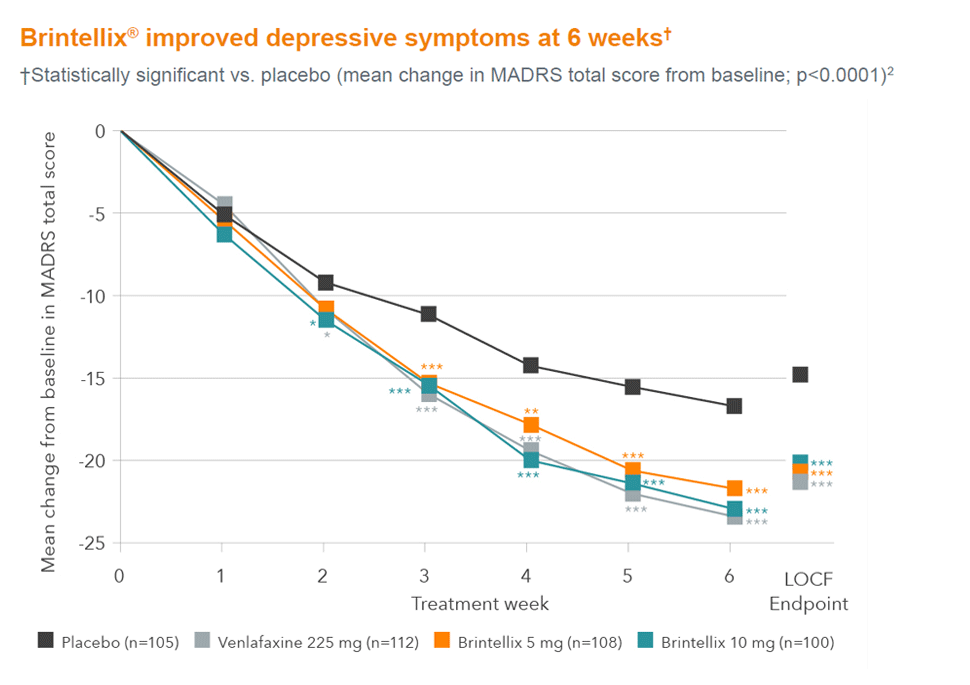
Graph indicates mean change from baseline in MADRS total scores (ANCOVA, FAS, on OC data over time) and LOCF (week 6); *p<0.05; **p<0.01;
***p<0.001 vs. placebo. Venlafaxine was included as active reference for study validation, not for comparison of effect size.1
Adapted from Alvarez et al. 2012.2
MADRS (Montgomery Asberg Depression Rating Scale)
FAS: Full Analysis Set
OC: (Observed Case)
LOCF (Last observation carried forward)
Study Design: A multicentre, randomised, double-blind, placebo controlled, active reference study involving 429 patients with MDD recruited
from 11 countries. Patients received Brintellix® 5 mg or 10 mg, placebo or venlafaxine XR (75 mg/d for first 4 days, 150 mg/d for the following
3 days, and then 225 mg/d for the remainder) once daily for 6 weeks. All patients had a baseline MADRS total score ≥30.2
Primary Endpoint: Change in MADRS total score from baseline to week 6.2
Graph indicates mean change from baseline in MADRS total scores (ANCOVA, FAS, on OC data over time) and LOCF (week 6); *p<0.05; **p<0.01;
***p<0.001 vs. placebo. Venlafaxine was included as active reference for study validation, not for comparison of effect size.1
Adapted from Alvarez et al. 2012.2
MADRS (Montgomery Asberg Depression Rating Scale)
FAS: Full Analysis Set
OC: (Observed Case)
LOCF (Last observation carried forward)
Study Design: A multicentre, randomised, double-blind, placebo controlled, active reference study involving 429 patients with MDD recruited
from 11 countries. Patients received Brintellix® 5 mg or 10 mg, placebo or venlafaxine XR (75 mg/d for first 4 days, 150 mg/d for the following
3 days, and then 225 mg/d for the remainder) once daily for 6 weeks. All patients had a baseline MADRS total score ≥30.2
Primary Endpoint: Change in MADRS total score from baseline to week 6.2
Adverse events that occurred in Brintellix® treated patients in the course of the MDD short-term, placebo-controlled studies with an incidence ≥10% were headache and nausea. Other adverse events include vomiting; diarrhoea; constipation; dizziness; generalised pruritus; hyponatraemia.1
For full information please see the Product Information.
