Brintellix® (vortioxetine) statistically significantly improves cognitive functioning (†) and functional capacity in patients with major depressive disorder (MDD) (1)
†Brintellix® demonstrated improved cognitive symptoms (DSST) and functional capacity (UPSA) in MDD (statistically significant vs. placebo, p<0.05).1
In this randomised, placebo-controlled, active-reference, double-blind, flexible-dose study on the efficacy of Brintellix® on cognitive function in MDD, Brintellix® statistically significantly improved everyday functioning in patients with MDD (p<0.001) as measured by the UPSA, which is a measure of ability to perform daily tasks.1
Explore the data in more detail below.
Change in UPSA composite score from baseline at week 81
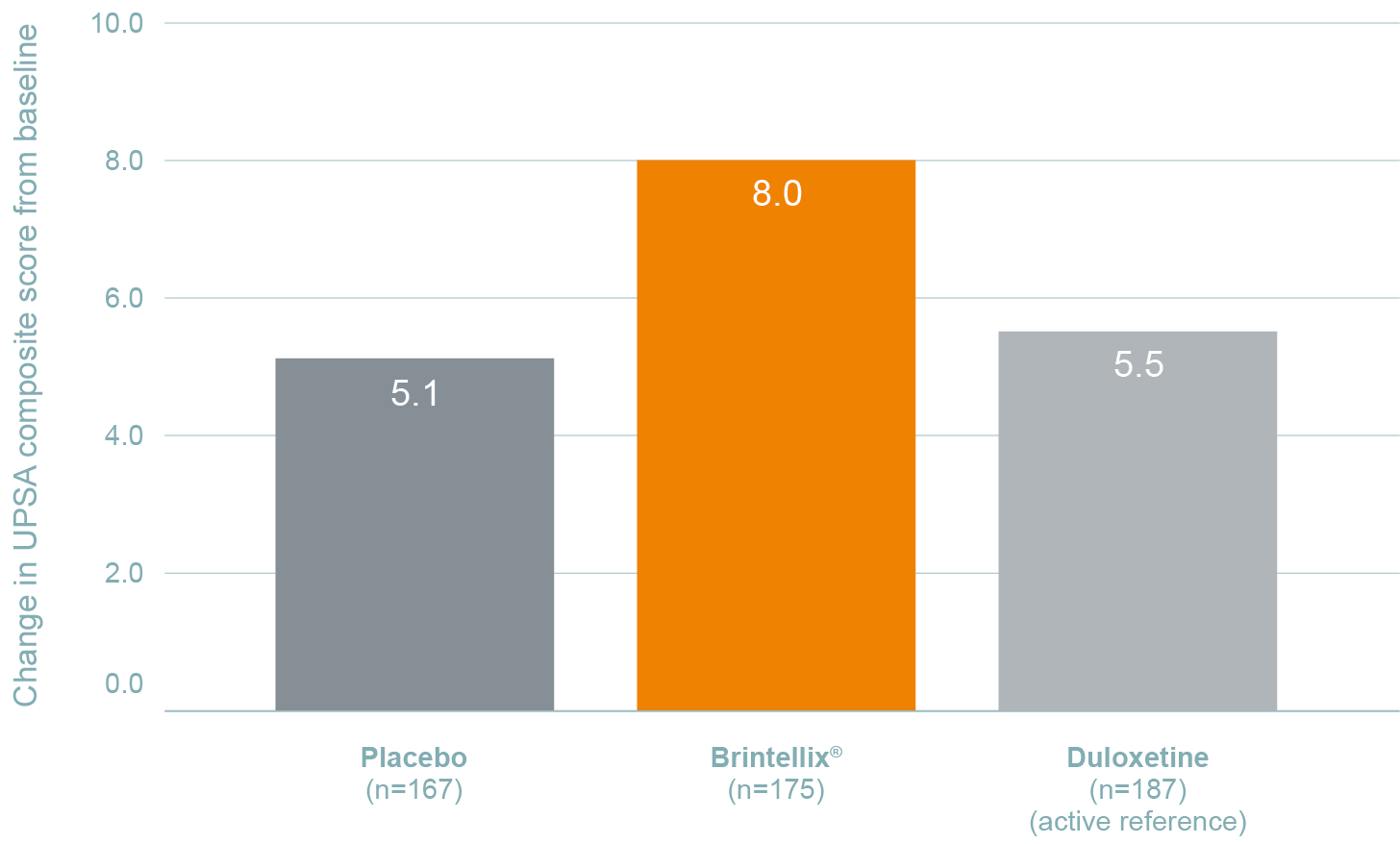
***p<0.001 vs. placebo; NS, not significant.
Duloxetine was included as active reference for study validation, not for comparison of effect sizes.
UPSA data presented are a composite of UPSA-VIM and UPSA-B.
Adverse events that occurred in Brintellix® treated patients in the course of the MDD short-term, placebo-controlled studies with an incidence ≥10% were headache and nausea. Other adverse events include: vomiting; diarrhoea; constipation; dizziness; generalised pruritus; hyponatraemia.2
For full information please see the Product Information.
Abbreviations
DSST, digit symbol substitution test; MDD, major depressive disorder; NS, not significant; UPSA, University of San Diego performance-based skills assessment; UPSA-B, UPSA–brief form; UPSA-VIM, UPSA–Validation of intermediate measures.
***p<0.001 vs. placebo; NS, not significant.
Duloxetine was included as active reference for study validation, not for comparison of effect sizes.
UPSA data presented are a composite of UPSA-VIM and UPSA-B.
Adverse events that occurred in Brintellix® treated patients in the course of the MDD short-term, placebo-controlled studies with an incidence ≥10% were headache and nausea. Other adverse events include: vomiting; diarrhoea; constipation; dizziness; generalised pruritus; hyponatraemia.2
For full information please see the Product Information.
Abbreviations
DSST, digit symbol substitution test; MDD, major depressive disorder; NS, not significant; UPSA, University of San Diego performance-based skills assessment; UPSA-B, UPSA–brief form; UPSA-VIM, UPSA–Validation of intermediate measures.
